Novo Nordisk A/S NVO announced that it is set to invest more than 16 billion Danish kroner (€2.1 billion) by the end of 2023 to expand the existing production site in Chartres, France, for the current and future product portfolio within serious chronic diseases.
Per the company, the investment is expected to significantly increase the capacity of the manufacturing site, adding aseptic and finished production processes and an extension of its current Quality Control Laboratory.
The expansion program is also a strategic move by Novo Nordisk to entrench its diabetes and obesity care market leadership by increasing the manufacturing capacity for its GLP-1 products, such as Ozempic and Wegovy. Notably, Wegovy has been witnessing exponential demand in the international market.
The announced investment plan is set to increase the company’s ability to meet future demands for Wegovy and other innovative medicines, thereby avoiding the loss of market share.
The pharma goliath further substantiated that the company’s expansion project will more than double the footprint of the site, upon completion. The new facilities incorporated in the manufacturing site will be designed as a multi-product facility to accommodate current and future processes, displaying state-of-the-art technology and a working environment.
At present, Novo Nordisk has initiated the construction projects, which will gradually be finalized from 2026 to 2028. The company expects to open job opportunities for more than 500 people to run production activities all day round when the construction is completed and the facilities are finalized.
Furthermore, during the construction phase of the expansion project, NVO anticipates employing up to 2,000 external workers.
Year to date, shares of Novo Nordisk have surged 52.6% compared with the industry’s 4.5% rise.
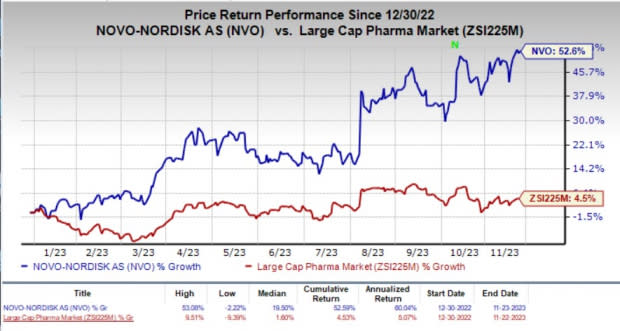
Image Source: Zacks Investment Research
Though a market leader in the Diabetes and Obesity Care industry, Novo Nordisk has started facing serious competition from other large-cap pharma companies that are also developing obesity drugs.
The obesity market has garnered much interest lately, ever since Novo Nordisk received FDA approval for Wegovy. The FDA approved Wegovy in 2021 for chronic weight management in adults with obesity or overweight.
We would like to remind the investors that, last week, Eli Lilly LLY announced its plans to spend $2.5 billion to build its first manufacturing facility in Alzey, Rhineland-Palatinate, Germany.
Per LLY, this new facility will boost global production of its parenteral (injectable) product and device manufacturing network as it expects growing demand for its marketed products, especially in the diabetes and obesity portfolio.
Lilly is also building the manufacturing site to ensure the safe and reliable supply of LLY’s innovative medicines. Earlier this month, the FDA approved tirzepatide, under the brand name Zepbound, for chronic weight management in adults with obesity or overweight. Tirzepatide was approved by the FDA last year under the trade name Mounjaro, a dual GIP and GLP-1 receptor agonist, to treat adults with type 2 diabetes mellitus (T2DM).
Since its launch, Mounjaro has shown an impressive initial uptake. Lilly recorded $2957.5 million in Mounjaro sales, in the nine months ended Sep 30, 2023. Before Zepbound’s approval, Mounjaro was being used off-label for weight loss. Approval of the obesity indication is expected to help Lilly rake in billions of dollars from tirzepatide sales.
Other pharma giants, like Pfizer PFE and AstraZeneca AZN, have also stepped into the obesity market. Pfizer is evaluating pipeline candidates for obesity. It is currently evaluating danuglipron in a phase II study in patients with obesity and T2DM. If this study is successful, Pfizer plans to start phase III studies by the end of the year.
Earlier this month, AstraZeneca announced that it has entered into an exclusive deal with Chinese private biotech Eccogene to develop the latter’s oral drug, ECC5004, for treating obesity, type-II diabetes and other cardiometabolic conditions. AZN plans to develop ECC5004 both as monotherapy and combination therapies.
With the deal, AstraZeneca will get exclusive global development and commercialization rights to Eccogene in all territories except China. In China, Eccogene and AstraZeneca have joint rights.
Novo Nordisk A/S Price and Consensus
Novo Nordisk A/S price-consensus-chart | Novo Nordisk A/S Quote
Zacks Rank
Novo Nordisk currently sports a Zacks Rank #1 (Strong Buy). You can see the complete list of today’s Zacks #1 Rank stocks here.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
AstraZeneca PLC (AZN) : Free Stock Analysis Report
Pfizer Inc. (PFE) : Free Stock Analysis Report
Novo Nordisk A/S (NVO) : Free Stock Analysis Report
Eli Lilly and Company (LLY) : Free Stock Analysis Report

